Severe Anemia in a Patient with Rheumatoid Arthritis: Differential Diagnosis and Hemolysis Workup
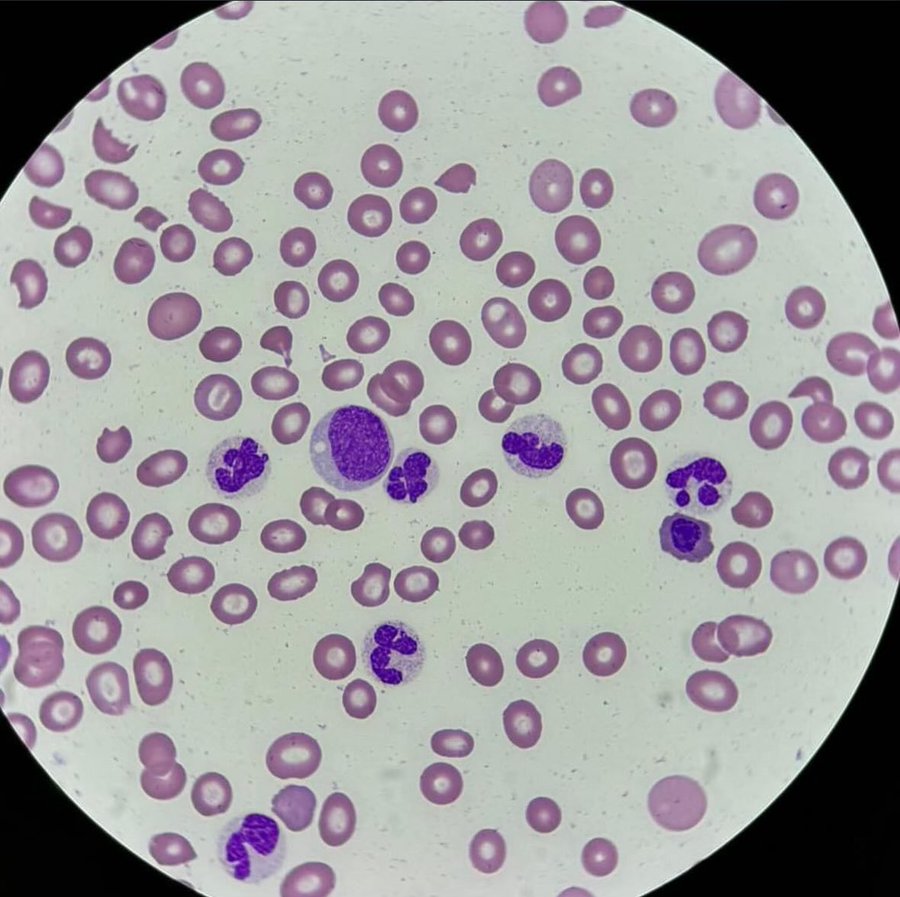
This peripheral blood smear shows significant red blood cell abnormalities, and given the context of rheumatoid arthritis (RA) and severe anemia, several possibilities arise for diagnosis:
In this case of a rheumatoid arthritis (RA) patient presenting with severe anemia, a peripheral blood smear reveals several diagnostic clues, including the presence of schistocytes and possible bite cells. Given the patient's history and findings, the differential diagnosis includes Microangiopathic Hemolytic Anemia (MAHA), methotrexate-induced folate deficiency, and G6PD deficiency, with Large Granular Lymphocytic Leukemia (LGLL) considered due to the association with RA. Further diagnostic testing including hemolysis labs and G6PD activity is crucial for accurate diagnosis.
Rheumatoid Arthritis with Anemia: RA is commonly associated with anemia of chronic disease, which usually presents with normocytic, normochromic anemia. However, given the severe anemia and additional findings, more specific causes need to be considered.
Large Granular Lymphocytic Leukemia (LGLL): As you mentioned, LGLL can be associated with RA and anemia. LGLL typically shows large granular lymphocytes, which may not be seen in this image. Absence of granularity in the cells makes LGLL less likely at this stage, but it is still worth considering if there are other clinical signs or peripheral lymphocytosis.
Folate Deficiency: Methotrexate (MTX), often used in RA treatment, can lead to folate deficiency, which causes megaloblastic anemia. However, the smear does not show hypersegmented neutrophils, which are characteristic of folate deficiency, making it less likely as a primary cause in this case.
Microangiopathic Hemolytic Anemia (MAHA): The presence of schistocytes (fragmented red blood cells) on the smear suggests the possibility of a hemolytic process such as MAHA. Causes include thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), or disseminated intravascular coagulation (DIC). MAHA should be considered, and appropriate labs (LDH, haptoglobin, bilirubin, and a Coombs test) should be done to evaluate hemolysis. The lack of platelets is concerning and suggests thrombocytopenia, which could be related to MAHA or another process.
G6PD Deficiency: The possible bite cell you mention could indicate oxidative damage, which might suggest G6PD deficiency, especially if the patient has been exposed to oxidative stressors (drugs, infections). This should be reviewed by checking the patient’s medication history and testing for G6PD activity.
Other Considerations: If hemolysis is confirmed, other causes of hemolytic anemia such as autoimmune hemolytic anemia (AIHA) should be evaluated. RA patients are also at risk for AIHA, especially if on immunosuppressive therapy.
Suggested Next Steps:
- Perform hemolysis labs: LDH, haptoglobin, reticulocyte count, indirect bilirubin, and Coombs test to rule out hemolysis.
- Investigate for MAHA: Check for schistocytes, and evaluate for TTP, HUS, or DIC.
- Consider G6PD testing if oxidative stress from medications is suspected (especially in the context of bite cells).
- Folate/B12 levels, especially given MTX use.
In summary, based on the smear and clinical context, you should focus on ruling out hemolytic anemia (MAHA) and medication-induced causes like G6PD deficiency and folate deficiency, given the patient's RA and treatment history.
Reference:
- Hoffman R, Benz EJ, Silberstein LE, et al. Hematology: Basic Principles and Practice. 7th ed. Philadelphia, PA: Elsevier; 2017. Available at: Hematology: Basic Principles and Practice.
© 2000-2025
Sieglinde W. Alexander. All writings by Sieglinde W. Alexander have a fife year
copy right.
Library of Congress Card Number: LCN 00-192742
ISBN:
0-9703195-0-9

Comments
Post a Comment